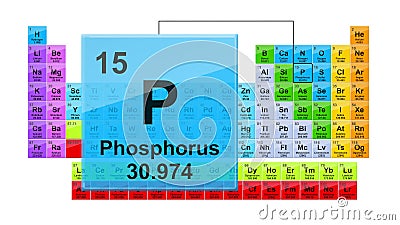
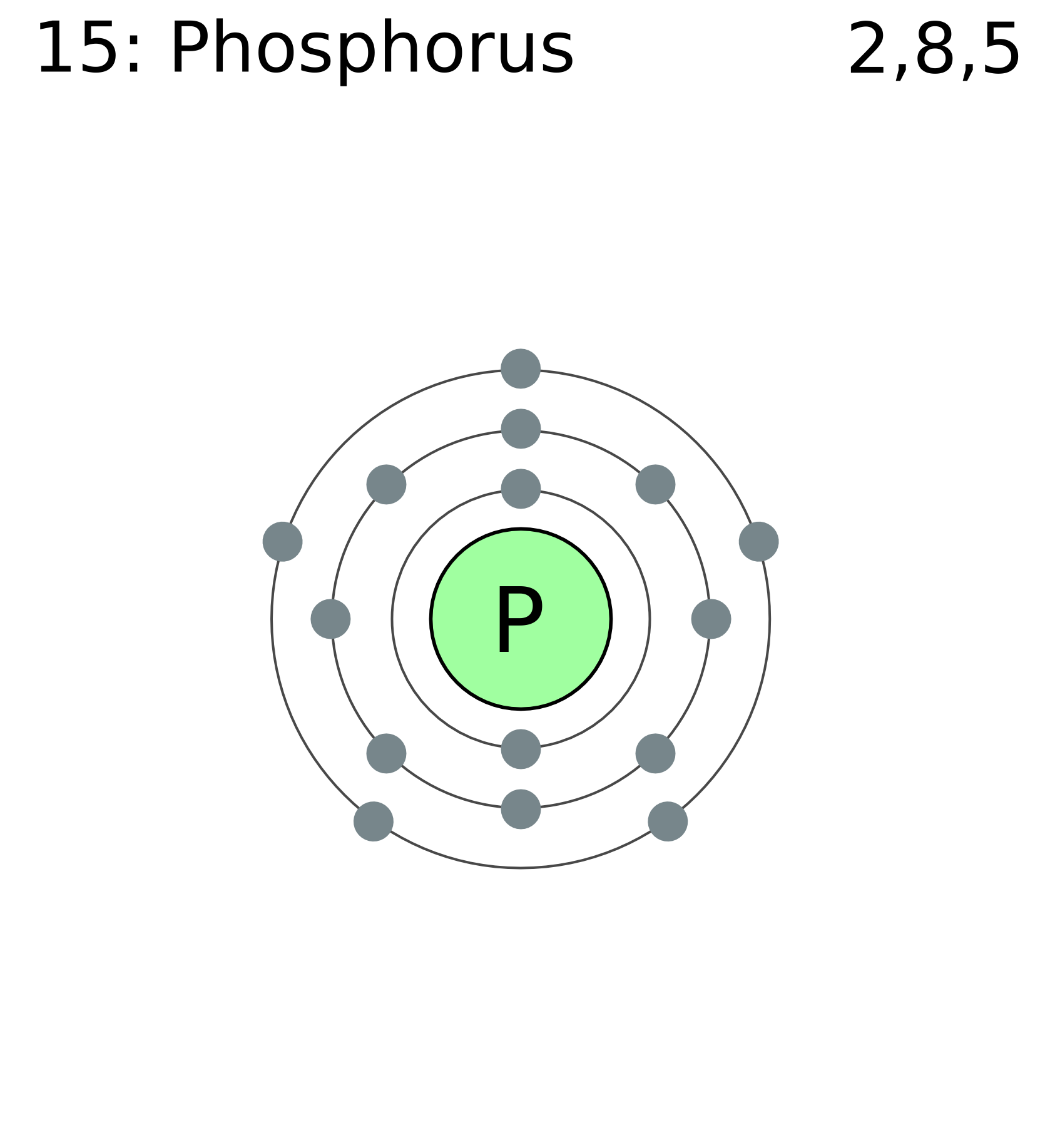
Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. The chemical symbol for Phosphorus is P. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but because it is highly reactive, phosphorus is never found as a free element on. While theoretical simulations predict contradictory results about how the intercalation of foreign metal atoms affects the order of atomic layers in black phosphorus (BP), no direct experimental visualization work has yet clarified this ambiguity. By in situ electrochemical sodiation of BP inside a high-resolution transmission electron microscope and first-principles calculations, we found. Phosphorus is a vital plant nutrient and its main use – via phosphate compounds – is in the production of fertilizers. Just as there are biological carbon and nitrogen cycles, there is also a phosphorus cycle. Phosphorus is used in the manufacture of safety matches (red phosphorus), pyrotechnics and incendiary shells.
- Atomic No Of Phosphorus Symbol
- Atomic No Of Phosphorus Steel
- Atomic No Of Phosphorus Vs
- Atomic No Of Phosphorus Stone
Atomic No Of Phosphorus Symbol
Atomic weight: Atomic weight values represent weighted average of the masses of all naturally occurring isotopes of an element. The values shown here are based on the IUPAC Commission determinations (Pure Appl. 73:667-683, 2001). The elements marked with an asterisk have no stable nuclides. Periods are the rows of the periodic table that from left to right increase in order of mass. As you go along periods the number of protons and neutrons increases.
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 31P | 30.973 761 998(5) | 1 |


Phosphorus is a monoisotopic element and its atomic weight is determined solely by its isotope 31P. The Commission last revised the standard atomic weight of phosphorus in 2013 based on the latest Atomic Mass Evaluation by IUPAP.
32P and 33P are cosmogenic and also can be produced by the nuclear industry, but their concentrations in normal materials aretoo small to affect Ar(P).
Atomic No Of Phosphorus Steel
Atomic No Of Phosphorus Vs

© IUPAC 2003
CIAAW
Atomic No Of Phosphorus Stone
Phosphorus
Ar(P) = 30.973 761 998(5) since 2013
The name derives from the Greek phosphoros for 'bringing light' because it has the property of glowingin the dark. This was also the ancient name for the planet Venus, when it appears before sunrise.Phosphorus was discovered by the German merchant Hennig Brand in 1669.
